|
Integrated Dilution calculator - during input in all tables, immediate calculation of : c[mol/l] from m[g] and vol[ml], and vice versa.
pH, osmolarity and specific conductivity in multi component buffers: The number of components in a buffer is unlimited.
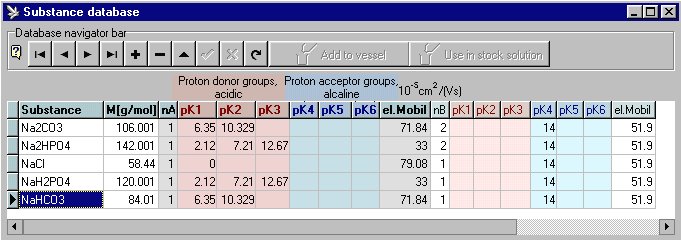
Integrated, enlargeable database: The database contains already a big number of Good's buffers, amino acids, organic and inorganic acids and bases and can be enlarged and modified by the user.
Automatic buffer selection for given pH. With a click in a pH-scale, the software automatically selects an optimal buffer substance from the database and adds it to the stock solutions table.
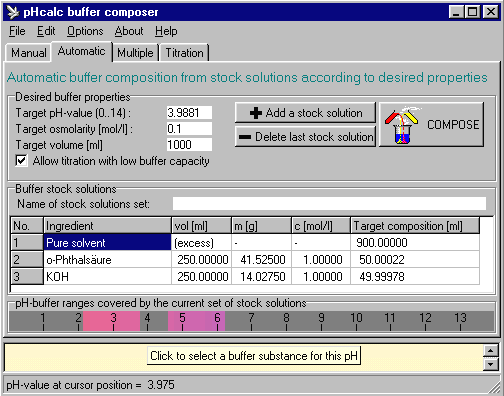
Automatic buffer composition according to given pH and salt content. You define the target pH and -osmolarity. The software checks the stock solutions set for the optimal buffer substance and titrates it with an additional acid or base to the desired pH and salt content. The needed volumes of the stock solutions are displayed together with the related masses and molar amounts of the stock substances to yield the buffer.
Serial buffer calculation: The calculation of the composition of a serial buffer (Example: "make five buffers between pH 5 and 7") is done with a few mouseclicks and the "automatic buffer composition".
Calculation of a pH-gradient microplate: The automatic buffer composition can be applied on user definable microplates. For each well in the microplate, the software selects the optimal buffer substances from the set of stock solutions. The results are saved in EXCEL-compatible text files.
Graphic titrations simulation: Your buffer can be titrated with one or two stock solutions, the result (pH as f(VolA, VolB)) is displayed in a graph. By mouse pointer, for each point of the tiration curve the pH value as well as the total volume and the concentrations and volumes of the titration reagents are displayed.
Languages English/German: By software-option, you can switch between english and german language.
Modular add-ons: On request, the software will be adapted to your special requirements (i.e.: connection with liquid handlers, balances etc...)
"Accuracy" of the calculations: The accordance of the calculations with a pH-measurement - depends not only on the correctness of the pKa values used, but also on the deviation of separate ion species from the idealised theoretical behaviour - and, on the concentration range. For most of the NIST-standard buffers (~0.05 M) the differences are found to be between 0.004 to 0.03 pH units.
|
|